|

|
The accuracy, completeness and legibility of all relevant information on laboratory requisition forms at the time submission of the specimens to the laboratory will ensure:
Please Provide The Following Information On Laboratory Requisitions:
Specimens must be accompanied by a fully completed Laboratory Requisition signed and dated by the ordering clinician.
Patient identification on requisition and specimens must match exactly to avoid rejection of specimens.
Each specimen must be labeled with two unique patient identifiers (First and Last name and date of birth or Health Card Number) and date of collection. Please see Guidelines for Specimen Identification and Acceptance for time sensitive tests (e.g. therapeutic drugs), and tests that have serial blood draws (e.g. GTT) both the date and time of sample collection MUST be clearly indicated on the specimen(s) in addition to the two unique patient identifiers.
Specimens that do not meet criteria specimen identification, or other acceptance criteria, will not be processed. Please see Guidelines for Specimen Identification and Acceptance. Laboratory tests must be individually and clearly marked on the requisition. Ordering of tests by profile or panel is not acceptable under MOHLTC policy.
To avoid misinterpretation which may compromise specimen integrity, or lead to delays in testing, please use the same test name/terminology listed in our Laboratory Test Requirements. For any test not listed in our Laboratory Test Requirement Manual, please contact our Client Services Department at (416) 449-2166 for further information. For tests performed by the Ontario Public Health laboratory (OPHL), please refer to OPHL’s Specimen Collection Guide.
The patient is responsible for payment of tests not covered under Ontario Health Insurance Plan (uninsured tests). Patients should be made aware of the cost of the uninsured test(s) prior to collection of specimens. Please call our Client Services Department at (416) 449-2166 to confirm pricing for uninsured tests.
For patients requiring repeat testing, the initial requisition signed by the clinician is only valid for the period specified by the ordering clinician. In accordance with MOHLTC policy, repeat testing on individual patients can be ordered for a maximum of six months without a new requisition. A copy of the initial requisition will be retained at the Patient Service Centre where the original service was provided, for the period specified on the requisition.
“To follow” specimens (e.g. stool, sputum, 24 hour urine collection etc.) that require the patient to collect the samples at home, are accepted up to thirty days from the date of service on the original laboratory requisition. “To follow” specimens submitted to the laboratory beyond the thirty days require a new requisition signed by the ordering clinician.
Verbal requests for test(s) are discouraged; however, under special circumstances an exception may be made. A completed requisition signed by the ordering clinician should be forwarded to the laboratory as soon as possible.
Clinicians may order additional tests (“Add-on” tests) on stored samples when clinically indicated. Acceptance of requests for “Add on” test(s) is based on: sample availability, sample quantity, and sample integrity (e.g. age and suitability). The laboratory will forward the required documentation to the ordering clinician for signature.
Patient access to test results is possible only when the ordering clinician has indicated on the requisition that access is permitted. Patient access applies only to the requisition in question.
Ontario Laboratory Accreditation (OLA) program has developed stringent criteria and standards for Specimen Identification and Acceptance in accordance with good clinical practice. Each specimen must be labeled at the time and point of collection with the patient’s full name (or unique code number in the case of anonymous testing), one other unique identifier (e.g. date of birth, Health Card number, accession number or patient chart number). Specimen labels shall also include the date of collection, and for time sensitive tests, the time of collection. Strict adherence to proper pre-analytical procedures in clinician’s offices, Patient Service Centres and laboratories, is essential for:
Deficiencies due to improper or incomplete identification of specimens and/or requisitions may result in delayed or incomplete testing, or in most cases rejection of the specimen(s). This policy is fundamental to patient safety. Specimens that fail to meet acceptance criteria will be rejected. Such specimens will not be processed and will not be returned to the referring clinician’s office.
Exceptions will be made for specimens considered to be ‘irretrievable’ or ‘difficult to collect’. Please see Irretrievable/Difficult to Collect Specimens.
Specimens must be properly labelled for patient safety and to prevent delays in testing resulting from specimen rejection. Please see Guidelines for Specimen Identification
Please note the following additional points to ensure specimens meet acceptance criteria:
Special consideration will be given to irretrievable and difficult to collect specimens for which outright rejection may lead to a potential risk to the patient. The following list is not exhaustive but includes those specimens for which both clinician and the laboratory must pay special attention:
If the ordering clinician can be identified, the laboratory will provide notification that an unlabelled, irretrievable specimen has been received. Such specimens may be returned to the ordering clinician’s office to initiate further investigation and to seek possible resolution.
Under certain circumstances, when specimen integrity may be compromised due to a delay in processing, the laboratory may proceed with the testing. For such specimens reports will only be generated and released upon the request of the ordering clinician. A disclaimer will be included on the report clearly stating that the unlabelled specimen received by the laboratory was identified by the clinician and the test results should be interpreted with caution.
Coded specimens received with a unique alpha, numeric, or alpha/numeric identifier assigned by the clinician, together with a second unique identifier are acceptable for HIV testing.
Alpha Laboratories will supply colour coded sterile evacuated plastic tubes for blood collection to referring clinicians who perform phlebotomy in their office. To order vacutainer supplies please see Laboratory Supplies.
To determine the correct tube for each test, please refer to the alphabetical test listing in the Laboratory Test Requirements.
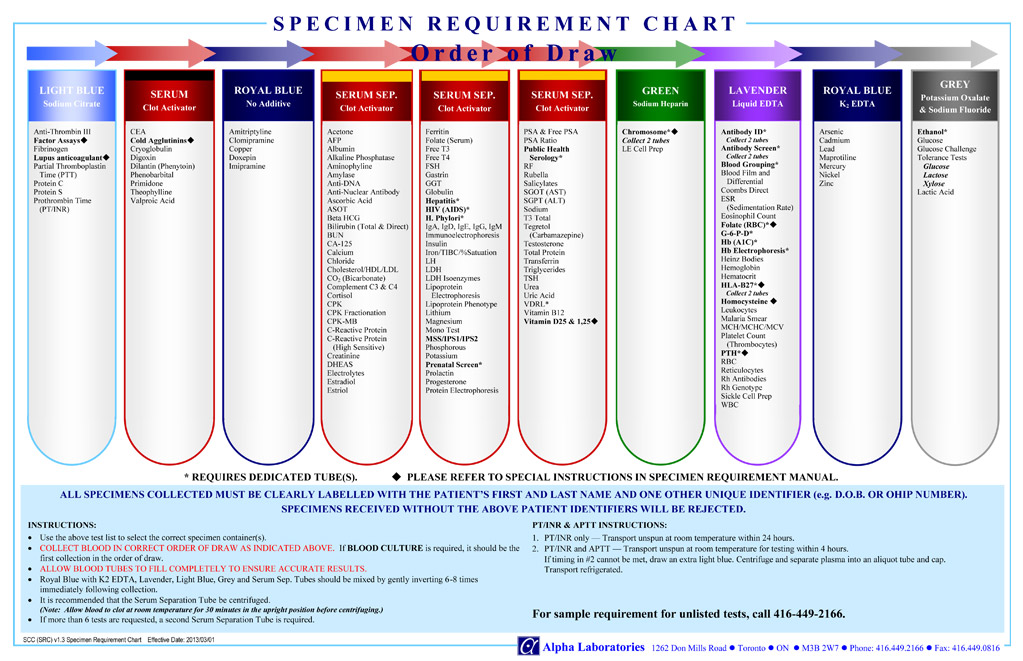
To confirm the order of vacutainer draw for blood collection please see Specimen Requirement Chart – Order of Draw Chart
All specimen containers must be clearly labeled with two unique patient identifiers: patient’s full name and one other identifier, (such as Date of Birth, Health Card Number or patient chart number), and date of collection.
Please also provide time of collection for time sensitive tests, and specimens requiring serial sampling (e.g. GTT).
Please note the following when using vacutainer tubes: